-
PDF
- Split View
-
Views
-
Cite
Cite
Florence Naillat, Renata Prunskaite-Hyyryläinen, Ilkka Pietilä, Raija Sormunen, Tiina Jokela, Jingdong Shan, Seppo J. Vainio, Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development, Human Molecular Genetics, Volume 19, Issue 8, 15 April 2010, Pages 1539–1550, https://doi.org/10.1093/hmg/ddq027
Close - Share Icon Share
Abstract
Germ cells are the foundation of an individual, since they generate the gametes and provide the unique genome established through meiosis. The sex-specific fate of the germline in mammals is thought to be controlled by somatic signals, which are still poorly characterized. We demonstrate here that somatic Wnt signalling is crucial for the control of female germline development. Wnt-4 maintains germ cell cysts and early follicular gene expression and provides a female pattern of E-cadherin and β-catenin expression within the germ cells. In addition, we find that Stra8 expression is downregulated and the Cyp26b1 gene is expressed ectopically in the partially masculinized Wnt-4-deficient ovary. Wnt-4 may control meiosis via these proteins since the Cyp26b1 enzyme is known to degrade retinoic acid (RA) and inhibit meiosis in the male embryo, and Stra8 induces meiosis in the female through RA. Reintroduction of a Wnt-4 signal to the partially masculinized embryonic ovary, in fact, rescues the female property to a certain degree, as seen by inhibition of Cyp26b1 and induction of Irx3 gene expression. Wnt-4 deficiency allows only 20% of the germ cells to initiate meiosis in the ovary, whereas meiosis is inhibited completely in the Wnt-4/Wnt-5a double mutant. These findings indicate a critical role for Wnt signalling in meiosis. Thus, the Wnt signals are important somatic cell signals that coordinate presumptive female follicle development.
INTRODUCTION
During primary sex determination in mammals, the somatic cells of the bipotential gonad are guided to follow either the female or the male differentiation pathway. The sex-related Y (Sry) gene located on the Y chromosome serves as one critical component for triggering testis development and functions especially as a determinant of the fate of the Sertoli cells (1). Sry triggers a cascade of gene expression that leads to the development of the secondary sexual characteristics that are typical of the male.
Wnt signalling and certain components of the Wnt signal transduction pathway are critical for female development (2–5). Wnt-4 in particular was identified as a key signal that promotes female development, since typical female characteristics are impaired in its absence and are replaced with some male ones. Consistent with these phenotypic changes, the somatic cells of the Wnt-4-deficient ovary express several genes ectopically that encode enzymes of the testosterone biosynthesis pathway (6).
Wnt signalling is thought to regulate female development via β-catenin (Ctnnb1) (7,8) and R-spondin (Rspo1) (4). Stabilization of β-catenin in the early testis, for example, induces some female characteristics (5,9), and β-catenin knock-out in the ovarian somatic cells shifts sexual development in the male direction (10).
The primary sex determination function of somatic cells is associated with concurrent commitment of the germline cells. The primordial germ cells are attracted to the indifferent gonad (11,12), and there the somatic cells nurse the germ cells, determine their sexual fate and promote their sexually dimorphic differentiation to either the sperm cells or the oocytes (11). In female mouse embryos, the germ cells undergo mitotic divisions until around E13.5 and then initiate meiosis, which continues to prophase I diplonema, a stage at which meiosis remains arrested until the start of ovulation (13). The germ cells in the embryonic testis undergo mitotic divisions and initiate meiosis only at puberty (14).
Retinoic acid (RA) controls the entry of the female germ cells into meiosis by inducing expression of a transcription factor, Stra8 (15,16). In contrast, in males, RA becomes degraded by the activity of the Cyp26b1 enzyme, and the initiation of meiosis is inhibited (14). Other than the RA pathway, the signals involved in the control of gonadal germ cell behaviour are poorly known.
We show here that Wnt signalling from the somatic cells of the developing ovary is critical for female germline development. Wnt-4 deficiency disrupts germ cell–cell contacts, and this is associated with a shift from a female- to a male-type pattern of β-catenin and E-cadherin (Cdh1) expression in the germ cells. Wnt-4 signalling is also critical for meiosis, since Stra8 and certain other meiosis markers are reduced in Wnt-4 deficiency. Wnt-4 may regulate meiosis via the RA pathway, as Cyp26b1 is ectopically expressed in the Wnt-4-deficient mesonephros–ovary unit, and expression is inhibited by Wnt-4 gain of function. Moreover, only ∼20% of the Wnt-4-deficient ovarian germ cells are able to enter into meiosis at E14.5, whereas meiosis is completely inhibited in the ovaries of Wnt-4/5a double-mutant embryos. We conclude that the fate of the female germline cells is under the control of somatic Wnt signalling.
RESULTS
Wnt-4 encodes a somatic ovarian cell signal
To determine whether Wnt-4 is expressed by the soma or the germline or both, we made use of the Wnt-4EGFPCre knock-in mouse line in which the Cre recombinase-encoding gene has been targeted under the control of the endogenous Wnt-4 promoter. The Wnt-4EGFPCre line was crossed with the floxed Rosa26LacZ reporter mouse in which the LacZ gene becomes permanently activated upon Cre-mediated recombination. The ovaries of the Wnt-4EGFPCre;Rosa26LacZ-positive newborn F1 mice demonstrated LacZ staining only in the ovarian somatic cells (Supplementary Material, Fig. S1A–C). Moreover, the pattern of LacZ expression in the F2 embryos that were derived from the Wnt-4EGFPCre;Rosa26LacZ-positive individuals expressing these cassettes resembled that of the endogenous Wnt-4 gene (Supplementary Material, Fig. S1D) (17). Hence Wnt-4 regulates female development as a somatic signal and it is not expressed in the female germline at any developmental stage.
Wnt-4 signalling has a role in the maintenance of the female germline cysts
To address the potential role of Wnt signalling in the development of the female germline cells, we analysed the presence of the germ cells in Wnt-4-deficient embryos. We found that germ cells, labelled with an antibody against germ cells expressed, Mvh (anti-Vasa homolog), were numerous in the Wnt-4-deficient ovary at E12.5 and E14.5 when the Kit ligand expression is already reduced (Supplementary Material, Table S1), but upon closer inspection, the germ cells failed to accumulate to the cortical part of the ovary (Supplementary Material, Fig. S2A–D, arrow). At E16.5, some germ cells were detected in close proximity to the mesonephros in the Wnt-4-deficient ovary. It was also noted at this stage that most of the female germ cell cysts had disintegrated to either single-germ cells or assemblies of a few germ cells only, whereas the corresponding wild-type germ cells remained clustered (Supplementary Material, Fig. S2, compare E with F, arrows and inserts) (12,13).
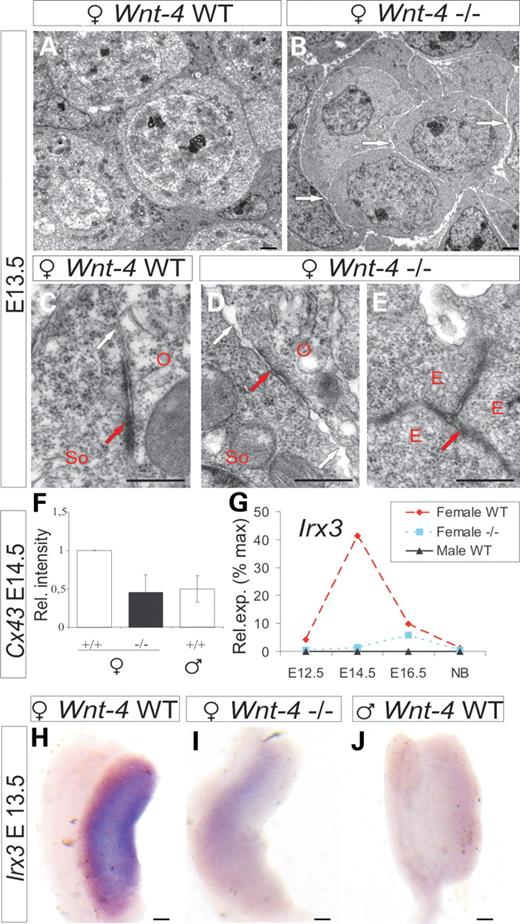
Ultrastructural analysis indicated that the germ cells in the Wnt-4-deficient ovary had become detached from each other and physical gaps were visible in the whole germ cell–somatic cell complex at E13.5, unlike wild-type control cells (Fig. 1, compare B with A and D with C, white arrows). The adherence junctions in developing oocytes and somatic cells (Fig. 1C, red arrow) were poorly organized in the case of Wnt-4 deficiency (Fig. 1D, red arrow), whereas these structures were readily detected in the endothelial cells of the emerging blood vessels in the same ovary (Fig. 1E, red arrow). Expression of Connexin 43 (Cx43) (18) and Iroquois3 (Irx3) (19) was downregulated in the Wnt-4-deficient ovary (Fig. 1F–J; Supplementary Material, Table S1). Hence Wnt-4 signalling is critical for the maintenance of cell–cell contacts within the developing female follicle.
Wnt-4 deficiency leads to impaired cell contacts between female germ cells and somatic cells. (A) The ultrastructure of germ cell–somatic cell complex of an ovary of a wild-type embryo. The cell contacts in the presumptive ovarian follicle are impaired at E13.5 due to Wnt-4 deficiency (B, D white arrows). An adherence junction has formed at the border region between a germ cell and a somatic cell in a normal ovary at E13.5 (C, red arrows) while the junction is defective in the case of Wnt-4 deficiency, and physical gaps between cells have formed (D, red arrow) while the junctions between the endothelial cells remain intact (E, red arrow). (F) Expression of Cx43 is reduced in the Wnt-4-deficient ovary at E14.5. qPCR (G) and whole-mount in situ hybridization (H–J) of Irx3 in wild-type or the Wnt-4-deficient ovary and testis. O, an oocyte; So, a somatic cell; E, an endothelial cell. Scale bar (A and B) 250 nm; (C–E) 500 nm; (H–J) 250 μm.
Wnt-4 deficiency shifts the β-catenin and E-cadherin expression patterns from the female type to the male type in the ovary
The compromised organization of the presumptive female follicle due to Wnt-4 deficiency may be caused by changes in expression of certain cell adhesion molecules such as β-catenin and E-cadherin. Wnt-4 signalling can target β-catenin to the plasma membrane in certain cells (8).
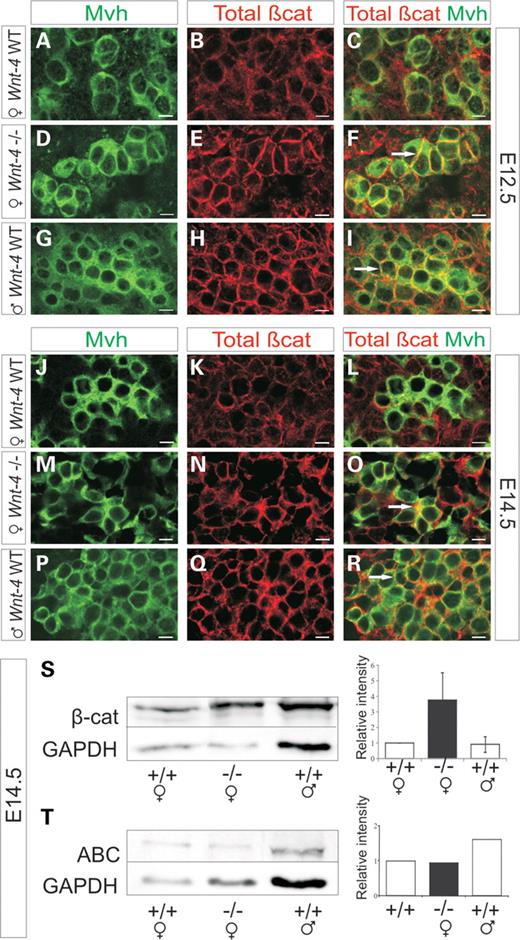
Most strikingly, a comparison of the dynamics of β-catenin and E-cadherin expression between embryonic ovarian and testicular germ cells revealed sexually dimorphic expression patterns. Typically at E12.5, β-catenin is weakly expressed throughout the female germ cell. In the case of Wnt-4 deficiency, it accumulated in the immediate borders of the germ cells instead, being similar in this respect to the expression pattern identified in wild-type male germ cells (Fig. 2A–I, compare F with I and C, in yellow, arrows). β-Catenin expression in wild-type female germ cells was reduced by E14.5 (Fig. 2J–L). However, in the Wnt-4-deficient ovary at E14.5, β-catenin expression still resembled the pattern identified for the wild-type testis of an E12.5 embryo (Fig. 2, compare P–R with G–I and M–O with D–F, in yellow, arrows).
β-Catenin expression pattern becomes shifted from the female type to the male type in the germ cells of the Wnt-4-deficient gonad. (A–C) β-Catenin (in red) is expressed throughout the Mvh+ germ cells (in green) at E12.5 in the ovary of wild-type female (C, yellow). (D–F) In the case of Wnt-4 deficiency, β-catenin accumulates at the sites of germ cell–cell contacts (F, in yellow, arrow). (G–I) The expression pattern in wild-type male germ cells is remarkably similar to that seen in the Wnt-4-deficient female germ cells (compare F with I, arrows), where β-catenin accumulates at the cell borders (I, in yellow, arrow). (J–R) Wnt-4-deficient ovarian germ cells at E14.5 resemble the pattern of the male and Wnt-4-deficient germ cells at E12.5 (compare O with I and F, arrows). (A, D, G, J, M and P) The germ cells identified with the Mvh antibody. The western blots demonstrating expression of the total β-catenin (S) and the non-phosphorylated, active form of β-catenin (T). Scale bar 100 μm.
E-cadherin, a cell adhesion molecule, is expressed in the ovarian germ cells until around E14.5, whereas its expression in the developing testis continues well beyond this stage (20). E-cadherin expression was unchanged at E14.5 irrespective of Wnt-4 deficiency (Supplementary Material, Fig. S3A–I), but became undetectable at E15.5 in the wild-type ovary (Supplementary Material, Fig. S3J–L), whereas it persisted in the Wnt-4-deficient germ cells in a manner comparable with that seen in the wild-type testis (Supplementary Material, Fig. S3, compare P–R with M–O and J–L).
Western blot analysis showed that the Wnt-4-deficient ovary expressed increased levels of β-catenin relative to the ovary of a wild-type embryo, but the stabilized form [anti-active β-catenin (ABC)] remained unchanged at E14.5 (Fig. 2S and T). E-cadherin expression was closer to the level observed in the wild-type testis in the Wnt-4-deficient ovary (Supplementary Material, Fig. S3S).
Since the transcription factors Gata4 and Fog2 are considered to contribute to the stability of the ovarian β-catenin, which is critical for female development (7), we analysed their expression in the Wnt-4 knock-out model. Although Gata4 expression was unchanged (Supplementary Material, Fig. S4A–G), unexpectedly Fog2 expression was upregulated compared with the controls due to Wnt-4 deficiency in the ovary (Supplementary Material, Fig. S4H and Table S1). Hence, the changes in the expression patterns of β-catenin and E-cadherin in the germ cells brought about by Wnt-4 deficiency demonstrate a shift from the female towards the male type.
Wnt-4 signalling is critical for the maintenance of the developing ovarian follicle
Given the fact that Wnt-4 represents a somatic cell signal in the developing female embryo (2) that also controls germline development, we analysed how the female properties of the early ovarian follicle would be maintained without Wnt-4 signalling. The Adamts19 gene, which is robustly expressed in the wild-type developing ovary but is not detected in the wild-type testis (21), was downregulated in Wnt-4 deficiency (Supplementary Material, Fig. S5A–C). Similarly, gene expression for the granulosa cell-derived Kit ligand (Kitl), the protein of which, together with the Kit receptor, controls the growth of the oocyte was downregulated (22) (Supplementary Material, Table S1). Moreover, the transcription factor Figla (Figα), which regulates the zona pellucida (Zp1-3) genes (23), was expressed at low levels in the Wnt-4-deficient ovary at E14.5 and newborn stages compared with the wild-type (Supplementary Material, Fig. S4I–N andSupplementary Data). Also, Nobox gene that controls oocyte gene expression and is essential for folliculogenesis (24) was downregulated in the case of Wnt-4 deficiency (Supplementary Material, Table S1). Expression of the transcription factor Foxl2 (25) did not change significantly (Supplementary Material, Fig. S4O), but expression of Gdf9 and Bmp15, both of which regulate the transition of a primordial ovarian follicle to a primary one (26), was reduced due to Wnt-4 deficiency (Supplementary Material, Table S1). Thus, Wnt-4 signalling is critical for the maintenance of the developing ovarian follicle.
Wnt-4 signalling controls germ cell entry into meiosis in the developing ovary
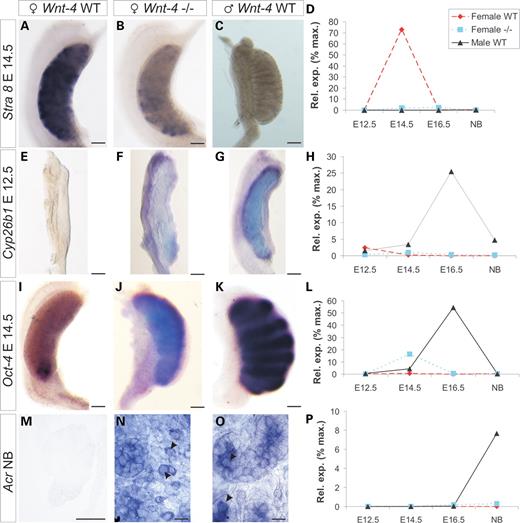
The germ cells initiate meiosis during E13.5–E15.5 (15,16,27). RA is a critical signal for the induction of meiosis (14) and regulates the entry via the promotion of ovarian Stra8 expression (14,15,28), which increases until E14.5 (Fig. 3A and D). Stra8 expression, which was not detected in embryonic testis (Fig. 3C and D) (15), was reduced in the Wnt-4-deficient ovary (Fig. 3B and D), suggesting that Wnt-4 signalling contributes to the initiation of meiosis in female germ cells.
Reduction in Stra8 expression and induction of Cyp26b1 expression in the Wnt-4-deficient ovary. Stra8 is expressed in the wild-type ovary at E14.5 (A), but expression is reduced in the Wnt-4-deficient ovary (B) while Stra8 is not expressed in the testis at this stage (C). qPCR profile of Stra8 expression (D). The Cyp26b1 gene is not expressed in the ovary (E) but it is expressed in the testis (G) at E12.5. Note that the Cyp26b1 gene has become expressed ectopically in the mesonephros of the Wnt-4-deficient embryo (F). qPCR of Cyp26b1 in the dissected embryos gonads (H). Oct4 is not detected in the wild-type ovary (I) but it is ectopically expressed in the Wnt-4-deficient ovary (J) being similar to the situation in the wild-type testis (K). qPCR of Oct-4. The Acrosin (Acr) gene is not expressed in the wild-type ovary (M) but it is ectopic in the Wnt-4-deficient germ cells (N, arrow heads). Acr is normally expressed in the germ cells of the testis (O, arrow heads). qPCR of Acr expression (P). Scale bar (A–K) 250 μm and (M–O) 100 μm.
The Cyp26b1 gene, which encodes an enzyme that degrades RA in the embryonic testis and, in this way, prevents male germ cells from entering into meiosis, is expressed in both sexes up to E11.5, but becomes restricted to the somatic cells of the testis by E12.5 (14). Consistent with the role of RA in meiosis, Cyp26b1 is not detected in the wild-type ovary at E12.5 or E13.5 (Fig. 3E and H; Supplementary Material, Fig. S5D), but is present in the testis at these stages and later (Fig. 3G and H; Supplementary Material, Fig. S5F). Interestingly, the Cyp26b1 gene was ectopically expressed in the Wnt-4-deficient mesonephros at E12.5 (Fig. 3F), but the expression shifted to the gonad by E13.5 (Supplementary Material, Fig. S5E, arrows). It is significant that the Wnt-4-deficient ovary also expressed reduced levels of Aldh1a1 and Aldh1a2, which are involved in the production of RA (14) (Supplementary Material, Table S1). The results support the conclusion that Wnt-4 signalling promotes the entry of the female germ cells into meiosis via the RA pathway that leads to the induction of Stra8 expression.
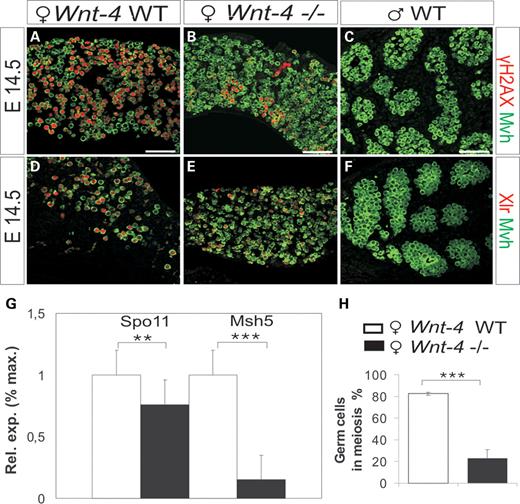
Stra8 has a function in the formation of the synaptonemal complexes (SCs) that are important for the progress of meiosis mediated by the SC proteins (15). We found that Sycp1 and Sycp3 expression was downregulated without Wnt-4 activity (Supplementary Material, Table S1), whereas Cpeb1, which encodes a protein that promotes meiosis by binding to the mRNA of Sycp1 and Sycp3 (26), was also reduced (Supplementary Material, Table S1). It should be noted that expression of Spo11 and Msh5, both of which regulate meiotic DNA repair proteins (29), also decreased in the Wnt-4-deficient ovary (Supplementary Material, Table S1; Fig. 4G).
A defect in meiosis in the developing ovary due to a lack of Wnt-4 signalling. (A–F) Antibodies against γH2AX and Xlr were used to identify induction of meiosis (in red), whereas the anti-Mvh antibody served to identify the germ cells (in green). At E14.5, germ cells have initiated meiosis and express γH2AX and Xlr (A and D) while their expression is reduced due to Wnt-4 deficiency (B and E). The male germ cells do not express these meiosis markers (C and F). (G) The expression of Spo11 and Msh5 genes at E14.5 is downregulated in the Wnt-4-deficient ovary (black bar) compared with the controls (white bar). (H) Around 20% of the germ cells in the Wnt-4-deficient ovary at E14.5 have the capacity to proceed in meiosis, whereas this value is close to 80% in the wild-type ovary at this stage. Scale bar 200 μm (***P < 0.005 and **P < 0.05).
Given these defects in expression of genes that are involved in the control of meiosis due to Wnt-4 deficiency, we next analysed potential changes in expression of meiosis markers and the total number of germ cells. The γH2AX and Xlr proteins were used as indicators of meiosis prophase I at E14.5, E15.5 and E16.5 (30,31). These were expressed in the germ cells of the wild-type ovary (Fig. 4A and D; Supplementary Material, Fig. S5G and J), but in the case of Wnt-4 deficiency, they were expressed only by some of the dispersed germ cells (Fig. 4B and E; Supplementary Material, Fig. S5H and K), whereas no expression was noted in the developing testis (Fig. 4C and F; Supplementary Material, Fig. S5I and L).
We counted the numbers of Mvh+ germ cells that were in meiosis and compared them with the total numbers of germ cells. Although ∼80% of wild-type germ cells had initiated meiosis at E14.5, only ∼20% of the Wnt-4-deficient germ cells had done so (Fig. 4H). Collectively, the findings point to a severe early defect in meiosis when Wnt-4 signalling is impaired.
A switch in the sexual identity of the female germ cells without Wnt-4 signalling
Since the β-catenin and E-cadherin expression patterns were masculinized in the Wnt-4-deficient ovary, we investigated whether the germ cell and soma might demonstrate additional male characteristics.
Lack of Oct4 (Pou5f1) function is associated with competence for entering meiosis in female embryos (27), whereas Piwil2 is crucial for spermatogenesis (32). Both of these genes were ectopically expressed in the Wnt-4-deficient ovary (Fig. 3I–L; Supplementary Material, Table S1).
Another male gene, Renin1 (33), showed a 14-fold induction in the Wnt-4-deficient ovary at E14.5, but was not expressed in the wild-type ovary (Supplementary Material, Table S1). Xmr, which is transcribed in the round spermatids at E14.5 (34), was also ectopically expressed in the Wnt-4-deficient ovary (Supplementary Material, Table S1). Strikingly, even Acrosin (Acr), which encodes a protein involved in fertilization (35) that was not expressed in the wild-type ovary (Fig. 3M and P), was present in some germ cells of the Wnt-4-deficient ovary (Fig. 3N, arrows) and the testis, (Fig. 3O, arrows, P).
Phase-contrast microscopic analysis revealed Wnt-4-deficient germ cells that had four nuclei at E14.5, unlike in controls (Supplementary Material, Fig. S6, compare A with B, C, arrowheads), suggesting a defect in cytokinesis. In addition, certain areas of the Wnt-4-deficient ovaries contained prospermatogonia-like cells that resembled a seminiferous tubule typical of the testis. Some poorly differentiated oocytes were also present in such ovary (Supplementary Material, Fig. S6, compare D–F with G–I, arrows). Thus, the Wnt-4-deficient ovary obtains male characteristics in both the somatic cells and the germ cells.
Reintroduction of Wnt-4 signalling partially feminizes the Wnt-4-deficient ovary
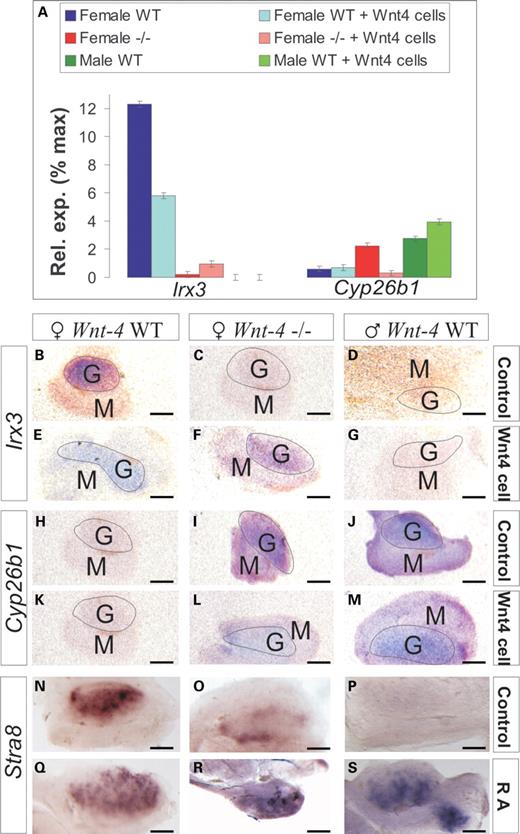
Since we had identified changes in certain sex-related parameters in the somatic cells and the germline in the Wnt-4-deficient ovary, we set out to examine whether reintroduction of the Wnt-4 signal in organ culture would rescue any of these. As the indicators, we selected the oocyte-somatic cell adhesion regulator Irx3 and the meiosis inhibitor Cyp26b1 (Fig. 5).
A partial rescue of the female properties of the Wnt-4-deficient ovary in response to the recovery of the Wnt-4 signal. (A–M) Irx3 and Cyp26b1 expression levels were analysed in ovaries and testes prepared from either wild-type or Wnt-4 mutant embryos at E12.0 after they had been co-cultured for 48 h with either the control NIH 3T3 cells or the cells expressing the Wnt-4. Changes in Irx3 and Cyp26b1 gene expression was analysed by qPCR (A) or by whole-mount in situ hybridization (B–M). The role of RA in the control of meiosis was analysed (N–S). Wnt-4 signalling induces Irx3 gene expression to a certain degree in the Wnt-4-deficient ovary compared with the unconjugated Wnt-4-deficient ovary. Wnt-4 inhibits expression of the Cyp26b1 in co-cultures with the Wnt-4+ cells. Co-culture of Wnt4+ cells with the Wnt-4-deficient ovary induces Irx3 expression (compare F with C), whereas this inhibits Cyp26b1 expression (compare L with I). RA promotes Stra8 expression in the wild-type ovary (compare Q with N), the wild-type testis (compare S with P) and the Wnt-4-deficient ovary (compare R with O). G, a gonad; M, mesonephros. Scale bar 100 μm.
When Wnt-4 was reintroduced into the embryonic gonad by combining a cell line that produced it, no notable changes in expression of Irx3 or Cyp26b1 was found in the wild-type testis (Fig. 5A, D, G, J and M), but Irx3 expression was induced to a certain degree in the Wnt-4-deficient ovary when compared with the untreated Wnt-4-deficient ovary (Fig. 5A, compare F with C, E and B). In contrast to this, Wnt-4 signalling inhibited Cyp26b1 expression in the Wnt-4-deficient ovary when compared with the untreated Wnt-4-deficient ovary (Fig. 5A, compare L and I with K and H), suggesting a role for RA in the process. This was tested in organ culture. Indeed, RA stimulated meiosis as judged by Stra8 expression in the germ cells of the wild-type testis and ovary (Fig. 5, compare S with P and Q with N) but also in the Wnt-4-deficient ovary (Fig. 5, compare R with O and N). Hence, Wnt-4 signalling appears to be connected to the control of the RA pathway.
Wnt-4 promotes meiosis in synergy with Wnt-5a signalling
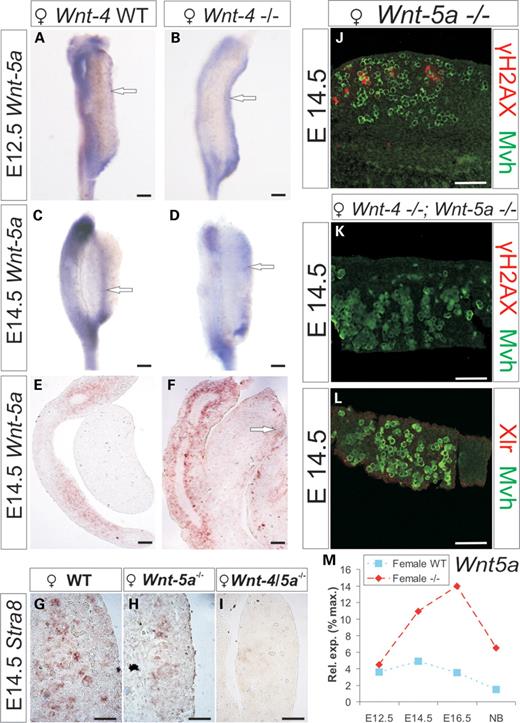
Since we found that ∼20% of the germ cells in the Wnt-4-deficient ovary still had the capacity to initiate meiosis (Fig. 4H), we speculated that Wnt-4 may function in concert with other Wnt factors to regulate meiosis. Indeed, Wnt-5a expression is upregulated in the Wnt-4-deficient ovary when compared with wild-type (Supplementary Material, Table S1; Fig. 6M) and confined to the cells under the germinal epithelium at E12.5 and E14.5 (Fig. 6A–F, arrows). Although the ovary of the Wnt-5a-deficient embryo expressed reduced amount of Stra8 and γH2AX (Fig. 6, compare H with G and J with Fig. 4A), strikingly, no expression of Stra8, Xlr or γH2AX were detected in the Mvh+ germ cells of the Wnt-4/Wnt-5a-deficient ovary at E14.5 (Fig. 6I, compare K and L with Fig. 4A and D).
Wnt-5a expression is induced in the Wnt-4-deficient ovary while meiosis is perturbed in the Wnt-4/5a-deficient ovary. (A) Wnt-5a is weakly expressed in the ovary at E12.5, but its expression is enhanced in the Wnt-4 mutant ovary in cells under the germinal epithelium (compare A with B, arrows). At E14.5, Wnt-5a expression is weak in the wild-type ovary, but expression is enhanced in the case of Wnt-4 deficiency (compare C and E with D and F, arrows). Stra8 is expressed in the wild-type ovarian germ cells (G) but expression is reduced in the Wnt-5a-deficient ovary (H). No Stra8 expression is detected in Wnt-4/Wnt-5a deficient ovary (I). Only few Wnt-5a-deficient ovarian germ cells express γH2AX (in red) at E14.5 (compare J to Fig. 4A) while the γH2AX or Xlr is not expressed in the Wnt-4/5a-deficient Mvh+ ovarian germ cells (compare K with L). (M) qPCR depicts Wnt-5a expression in the wild-type and the Wnt-4-deficient ovary. Scale bar (A–D) 200 μm; (E and F) 100 μm; ( G–I) 50 μm; (J–L) 100 μm.
DISCUSSION
Wnt-4 in the control of assembly of the presumptive female follicle
The sex-specific fate of the germline in mammals is thought to be controlled by somatic signals (11), which are still poorly characterized. We now provide evidence that Wnt-4 serves as a critical somatic cell component controlling the development of the female germline. Some of the germ cells of Wnt-4-deficient embryos fail to reach the ovarian cortical region, and the germline cysts composed of dividing germ cells (13) reduce prematurely to single cells or to assemblies of a few germ cells with physical gaps between them and the somatic cells. No such gaps are noted between wild-type germ cells and somatic cells, indicating a severe defect in the female germline cells caused by Wnt-4 deficiency. Since cell adhesion mediated by the gap junctional proteins and adhesion molecules is expected to be important for cystogenesis, and as we found a reduction in expression of Connexin 43 and Iroquois 3, both of which contribute to cell adhesion, the data support the conclusion that Wnt-4 function is important for the maintenance of female germline cysts.
Apart from the defects in the female germ cell cysts, we noted that the Wnt-4-deficient developing follicle also had other defects. For example, E-cadherin and β-catenin expression were localized differently in the germ cells of developing testis and ovary, and their expression patterns shifted from a female type to a male type when Wnt-4 function was impaired. More specifically, we found that E-cadherin expression persisted to later developmental stages in the mutant ovary and that this resembled the situation in the developing testis. β-Catenin also became localized to the immediate cell–cell contact sites in the Wnt-4-deficient ovarian germ cells, a pattern that was typical of the male germ cells. Hence, the Wnt-4-deficient germ cells became masculinized in this respect.
Wnt-4 in the maintenance of female germline characteristics
In addition to the afore-mentioned differences in the germ cells of the Wnt-4-deficient ovary, changes in other indicators also support a critical role for Wnt-4 as a promoter of female germline development. We found that ovarian follicular factors such as Figla, Nobox, Gdf9 and Bmp15 were all reduced in expression without Wnt-4 activity when compared with the situation in the wild-type ovary. On the basis of these results, we propose that Wnt-4 signalling regulates the development of the follicular complex during ovarian organogenesis by maintaining the female germline cell characteristics. It is also significant that some germ cells of the Wnt-4-deficient ovary had four nuclei, whereas this was not the case in wild-type germ cells, suggesting a defect in cytokinesis. The planar cell polarity Wnt signalling pathway controls cell polarity, which involves the PAR proteins and changes in the cytoskeleton (36), which are also critical for cytokinesis. Hence, Wnt-4 may also influence the germ cells by taking part in the control of cytokinesis at the same time.
Even though the details of the signal transduction of Wnt-4 during ovarian development remain to be demonstrated, Wnt-4 evidently signals synergistically with Rspo1 and the canonical Wnt signalling component β-catenin. Consistent with this notion, knock-out of either Wnt-4 or Rspo1 function leads to partial female-to-male sex reversal (2,4). Since our results demonstrate an accumulation of β-catenin between adjacent germ cells in the case of Wnt-4 deficiency, we speculate that the function of somatic Wnt-4 may be dualistic. First, Wnt-4 signalling may take part in controlling the distribution of β-catenin in the cytoplasm by promoting its localization to the membrane where E-cadherin is localized to have an input to cell adhesion, for example, as in other models (4). Since we found male-type accumulation of β-catenin in the plasma membrane of the germ cells in the case of Wnt-4 deficiency, other factors normally inhibited by Wnt-4 in the ovary apparently influence the localization of β-catenin in the partially masculinized ovary. Rspo1 signalling involves β-catenin (4) and may, like Wnt-4, as shown here, influence the localization of β-catenin within the germ cells since β-catenin also accumulates at cell–cell borders in the germ cells of Rspo1-deficient female embryos (4). The mechanisms of this warrant further investigations.
Second, Wnt-4 may coordinate the amount of β-catenin that enters the nucleus in order to regulate the Wnt target genes by means of the TCF proteins to take part in the regulation of oocyte differentiation. We conclude that Wnt-4 plays a role in promoting female-type expression patterns of β-catenin and E-cadherin in the germ cells in order to regulate follicle development.
Wnt-4 as a signal involved in the control of meiosis in the ovary
One unique feature of the female germline cells in the mouse is that they initiate meiosis around E13.5. Meiosis is thought to be initiated by RA signalling, leading to the induction of expression of a transcription factor, Stra8 (28). In the male embryo, meiosis is inhibited by the enzyme Cyp26b1, which degrades RA, contributing to the quiescence of the male germ cells (37). Since β-catenin function is not critical for the initiation of meiosis but is essential for the subsequent survival of the meiotic germ cells (10), Wnt-4, as a ligand located further upstream, may have a broader signalling outcome that targets the control mechanisms of meiosis. Consistent with this idea, we found that Stra8 expression is reduced in the Wnt-4-deficient female while Cyp26b1 becomes ectopically expressed; the introduction of a Wnt-4 signal into the Wnt-4-deficient ovary by means of a cell line expressing Wnt-4 inhibited the ectopic male-type Cyp26b1 expression. Also, RA promoted meiosis in the Wnt-4-deficient ovary as judged by Stra8 expression. Hence, Wnt-4 may have a role in the control of RA signalling, which may differ from the roles how Rspo1 is involved in the control of meiosis. In the zebrafish, for example, Wnt signal transduction controls an enzyme Cyp26a1, which is related to Cyp26b1 and is also involved in the catabolism of RA (38). Besides these points, it is also worth noting that the reduction in E-cadherin expression in females is normally associated with the initiation of meiosis (20). Since we found upregulation of E-cadherin expression in the Wnt-4-deficient female germ cells, we consider this yet another trait that points to a role for Wnt-4 in the control of meiosis. Meiotic germ cells normally suppress sex cord formation and in this way inhibit the masculinization of the somatic cells and germ cells (3). Consistent with these results, we found spermatogonia-like cells within a sex cord-like structure, thus resembling the seminiferous tubules depicted in the anterior part of the Wnt-4-deficient ovary. Thus, impaired meiosis due to the Wnt-4 deficiency could be another factor contributing to the masculinization of the Wnt-4-deficient ovary.
In support of the key role of Wnt signalling in meiosis, Rec8 and Syp3 were downregulated in the Wnt-4-deficient ovary. The diminished expression of these components may be expected to make a further contribution to the failure of meiosis caused by Wnt-4 deficiency, possibly by being not properly loaded onto the chromosomes (28). Consistent with the role of Wnt-4 signalling in the initiation of meiosis, we found that ∼80% of the Wnt-4-deficient germ cells failed to enter meiosis, as judged from the Xlr and γH2AX markers. Also, neither meiotic germ cells nor Stra8 expression was detected in the Wnt-4/5a double-mutant ovary in a situation where the germ cells were still present. Stra8 expression was also reduced in the Wnt-5a-deficient ovary. We may conclude on the basis of these findings that Wnt-4 regulates meiosis in synergy with Wnt-5a. Collectively, the data suggest that Wnt-4 signalling promotes the initiation of meiosis by influencing the RA pathway and the activation of Stra8.
In addition to Wnt-4, which controls female germline development, Nanos2 is in turn critical for male germ cell sex determination. If Nanos2 is ectopically expressed in the ovary, it prevents meiosis, making it one critical factor for the induction of germ cells to follow the male germ cell differentiation pathway (39). For these reasons, ectopic Nanos2 expression can be regarded as a candidate contributor to the repression of meiosis in the partially masculinized Wnt-4-deficient ovary. How the Wnt-4 signalling pathway interacts to inhibit Nanos2 function in females warrants further investigations.
All told, Wnt-4 is critical for female germline development, since only 20% of the Wnt-4-deficient germ cells have the capacity to undergo meiosis and/or continue in the process, whereas meiosis is completely inhibited in the ovary of Wnt-4/5a double-mutant embryos. The results indicate a role for the Wnts as somatic cell signals in female germline development, regulating the behaviour of the latter. The data obtained from both loss-of-function and gain-of-function studies point to a model in which Wnt signalling influences germline development by patterning β-catenin and E-cadherin and meiosis through the inhibition of Cyp26b1 expression, which allows the production of RA and entry into meiosis via enhanced expression of Stra8 (Supplementary Material, Fig. S7).
MATERIALS AND METHODS
Mouse lines
The Wnt-4, Wnt-5a knock-out and Wnt-4EGFPCre knock-in mice were maintained and genotyped as reported previously (2,6,17,40). The gonads of the wild-type (Wnt-4+/+) littermate embryos served as controls. All the experiments involving mice were approved by Finnish national legislation, the European Convention (ETS 123) and EU Directive 86/609/EEC.
RNA extraction and quantitative real-time PCR analysis
Organs that were prepared from embryos that had inherited the same targeted alleles were pooled for RNA extraction and PCR. Eight pairs of isolated gonads for each genotype were pooled at E12.5, six pairs at E14.5 and one pair at E16.5. The RNeasy kit was used for the purification of RNA (Qiagen), and the microarrays and their analyses were performed as described (6).
A total of 1 μg of RNA was reverse-transcribed into cDNA using the First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's recommendations. The TaqMan® Gene Expression Set-up, reaction conditions and reagents from Applied Biosystems were used to identify changes in expression of the Acr, Cyp26b1, Irx3, Oct4 and Stra8 genes. The ABI 7500 Applied Biosystem quantitative real-time PCR (qPCR) analysis equipment was used. In order to identify the potential changes in expression of the Fog2, Foxl2 (7), Msh5 and Spo11 genes, 2 μl of cDNA in combination with 0.3 μm of the respective primers and the Brilliant SYBRGREEN qPCR master mix, all from Stratagene, were employed. The primers used to analyse Msh5 and Spo11 gene expression were Msh5L: gagccaaagggacctgaaat; Msh5R: cgctgtttgcttatctctgga; Spo11L: gaagtgcctgccttcacaat; Spo11R: gccgacagaatcatcaaacat. The MX 3005 P sequence-detection system (Stratagene) was used for these primers. The Gapdh gene served in all cases as the reference. The assays were conducted from pooled gonads in triplicates. The values were analysed with Student's t-test, and the qPCR results are presented as means ± the standard error. The standard error was 0.2, and the statistical significance was defined as P < 0.02. The graphs of the qPCR shown in the results section are presented so that each value at a specific developmental stage was scaled to the maximum value obtained for each given gene.
In situ hybridization
The whole-mount and non-radioactive section in situ hybridization techniques were performed as described (6). The cDNA probes were obtained as gifts from the following persons: Cyp26b1, Adamts19 (David Page), Stra8 (Peter Koopman), Irx3 (Michel Michaud), Figla (Asma Amleh), Gata4 and Oct-4 (Hans Schöler) and Wnt-5a (Andy McMahon). The probe recognizing Acr was an EST (3984550). A minimum of three wild-type and three Wnt-4-deficient gonad pairs were examined for each gene and developmental stage.
Culture of the gonads and the Wnt-4 expressing cells
Generation of a cell line that expresses the Wnt-4 protein and the tests for the functionality of this cell line by means of induction of kidney tubules were as described (41). Wnt-4-expressing and normal control NIH3T3 (41) cells were grown to 70–80% confluence in DMEM (Gibco) supplemented with 10% of fetal calf serum (Sigma) and 0.1% of antibiotics penicillin–streptomycin (Sigma), and aliquots of these cells (1.20 × 106 cells/ml) were separately transferred to small pieces of nucleopore filters with the pore size of 0.1 μm and the cells were allowed to attach for 4 h (41). Thereafter, a second filter was placed on top of the cells with the embryonic mesonephros/gonad blocks that were prepared at E12.0 from the Wnt-4-deficient or control embryos. In the experiments, the trans-RA of 1 μm (Sigma) was used, and the mesonephros/gonad blocks were overlaid on a nucleopore filter and cultured as indicated (41). After 48 h of culture, the gonads were snap-frozen in liquid nitrogen for the extraction of RNA or fixed with 4% paraformaldehyde (PFA) and processed for whole-mount in situ hybridization as described (6). After 48 h of co-culture, the gonads were removed from the filters and either frozen immediately in liquid nitrogen to purify RNA or fixed with 4% PFA to process the samples for whole-mount in situ hybridization analysis.
Western blotting
Two pairs of testis or ovary were prepared at E14.5, and the proteins were separated by 10% SDS–PAGE as described (42). The primary antibodies were anti-active β-catenin (ABC, Lake Placid, NY, USA), anti-β-catenin (BD Transduction Laboratories), anti-Connexin 43 (Zymed), anti-E-cadherin (BD Transduction Laboratories) and anti-glyceraldehyde-3-phosphate dehydrogenase, clone 6C5 (GAPDH) (Chemicon International), used in the amounts suggested by the manufacturers. The appropriate HP-conjugated secondary antibodies obtained from Jackson ImmunoResearch Laboratories Inc. were applied in the dilutions recommended by the producer. The LumiGLO Kit (Cell Signalling Technology, Inc.) was used to identify the antibodies as described (42). The membranes were analysed with a Fuji Film Luminescent Image Analyzer LAS-3000. The separated protein bands were quantified with the Quanty One software (BioRad) and normalized to GAPDH. The analysis was done in triplicate and the results are presented as means ± standard deviation.
β-Galactosidase assay, immunohistochemistry, squash preparations and phase contrast microscopy
β-Galactosidase activity was visualized in the sections as published (17). For immunohistochemistry, testes and ovaries prepared at the stage indicated in the Results section were immersed in a 30% sucrose solution for 15 min, mounted, cut and stained with mouse anti-XLR antibody obtained as a kind gift from Denise Escalier or with anti-γH2AX antibodies (Millipore), whereas the germ cells were revealed with rabbit anti-Vasa homolog (Mvh) antibody (Abcam). The corresponding Alexa Fluor-conjugated anti-mouse and anti-rabbit antibodies (Invitrogen) were used.
To identify the degree of meiosis in the female germ cells, four pairs of ovaries from wild-type and Wnt-4-deficient embryos were prepared, sectioned and stained with anti-Mvh antibody, whereas those germ cells that had initiated meiosis were identified from the same samples with the anti-γH2AX or anti-Xlr antibodies and secondary antibodies that did not cross-react with the anti-Mvh antibodies. The pictures were taken with an Olympus Fluoview FV1000. The germ cells that were positive for Mvh and γH2AX and/or Xlr were counted with the aid of the Olympus FV10-ASW software. The percentage of the calculated germ cells that were in meiosis was defined. The values were analysed with Student's t-test, and the results are presented as means ± the standard deviation.
The squash preparations were made by dissecting the embryonic ovaries in ice-cold Dulbecco's phosphate-buffered saline, pH 7.3, placed onto a slide and squashed with a cover slip immediately prior to viewing with a Zeiss/Anxiovert 405M phase-contrast microscope. The images were taken with a Photometrics Cool SNAP HQ camera (USA).
Transmission electron microscopy
Micro-surgically prepared ovaries were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 m phosphate buffer, pH 7.4, post-fixed in 1% osmium tetroxide, dehydrated in acetone and embedded in Epon LX 112 (Ladd Research Industries, VT, USA). Semi-thin sections (1 μm) were first stained with toluidine blue for light microscopic inspection. Thereafter, thin sections (80 nm) were cut with a Leica ultracut UCT ultramicrotome, stained in uranyl acetate and examined in a Philips CM100 transmission electron microscope. Images were captured with a CCD camera equipped with TCL-EM-Menu, version 3, from Tietz Video and Image Processing Systems GmbH (Gaunting, Germany).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported by the grants from the Academy of Finland (206038, 121647), the Sigrid Jusélius Foundation and the European Union (LSHG-CT-2004-005085; HEALTH-F5-2008-223007 STAR-T REK).
ACKNOWLEDGEMENTS
We thank H. Härkman, J. Kekolahti-Liias, J. Kujala, M. Matero and J. Vuoristo for their excellent technical assistance. We are grateful to M. Heikkilä and F. Pujol for fruitful discussions.
Conflict of Interest statement. None declared.
REFERENCES
Author notes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.